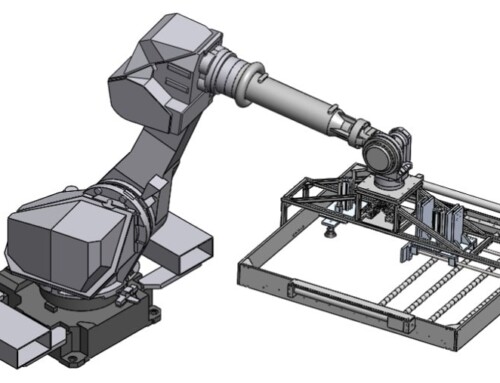
The Client is a global life-sciences company who wanted to make a Medical Device which works as an administering device for delivering a therapeutic substance in a fluid to a patient.
This Contract Design and Manufacturing Project had significant design complexities as mentioned below:
- A splash proof design was necessary to prevent the therapeutic substance from affecting the operators as well as the device electronics.
- The therapeutic substance is supplied in a syringe which needed to be continuously agitated (CW & CCW) at a precise angle with a fixed rotation speed to ensure a uniform dispersion during administration.The syringe is connected to rotary fittings and tubing. Therefore, it was imperative that the agitation movements do not twist or damage the syringe and tubing accessories.
- The therapeutic substance is administered via an infusion line with a dispensing force of around 150N (15kg.f). Operators are also required to dose in small quantities and at low speeds (around 1cc/min). So, the dosing mechanism required high axial force at slow speeds and a very smooth movements with no vibrations.Scale markings, precisely calibrated to be the same specifications as the syringe markings were required to give the operator an idea of dosing volumes.
- Overdosing a patient can be very serious. An overdose prevention mechanism was required to prevent the dosing mechanism from compressing the syringe further. For operational safety, it was required to protect the the overdose prevention mechanism from getting damaged due to intentional over-load.
Our Solution


- This design incorporates near all types of drive mechanisms like a belt & pulley drive, rack & pinion unit, spur gears, miter gears, worm gears and a precision ball screw.
- Two pinions on a single rack interlock to provide a locking function which acts as a overdose prevention mechanism.
- A custom-designed bi-directional slip clutch allows the operator to move the drive forwards and backwards with minimum torque. When the drive overloads, the slip clutch (tuned to a specific load capacity) slips gently to indicate an overload.
- A stainless steel shim runs thru the sliding blocks to prevent droplets from flowing into the electrical enclosure.
- A thick acrylic part and an aluminum housing provide shielding and safety. A Polycarbonate cover further helps with shielding and impact resistance.
- An ISO13485 certified DC gear motor provides a low noise fixed agitation speed and two fork sensors act as limit switches to change the direction of rotation.
- The electric circuit (PCB) & AC-DC converter is inside a metal perforated enclosure, which helps with safety shielding as well as electrical protection.
- The Plastic Enclosure has been injection molded with ABS UL94 V0 & Polycarbonate UL94 V0.
- A custom-designed packing box was made with cardboard and foam and it passed the IEC testing including the 1m drop test.
The Result
Once the design was finalized, the detailed documentation (design drawings, manufacturing instructions, Quality Control Documents) were approved and locked down by the client. A Pilot Build for Safety Studies was done as per the ISO 13485 standards and procedures. A manufactured units and corresponding batch records (copy) were delivered to the Client. The Medical Device is currently under-going safety studies followed by certification thru the various medical authorities before volume manufacturing commences.